We continue to improve our IT solutions for organizing and conducting clinical trials (CT), while remaining open to sponsors. Our goal is to increase the sponsor’s confidence that the study is proceeding as planned.
IPHARMA has developed a new service that allows employees of sponsor companies to monitor the progress of clinical trials. The platform is integrated with the system of electronic collection and analysis of participant data and provides information on participant enrollment, undesirable and serious adverse events, responses to inquiries and completion of individual registration cards.
The service is called “Sponsor’s Dashboard” and is available as a one-stop shop for quick and convenient access to study data. Employees of the sponsoring companies can track up-to-date information and promptly respond to any changes.
The platform provides an opportunity to view data in both raw and aggregated formats. This makes it possible to use them for internal corporate reports and to provide them to the company’s management.
Data in raw format represent raw information obtained from an electronic data collection system. They can be useful for detailed analysis and understanding of specific aspects of the study.
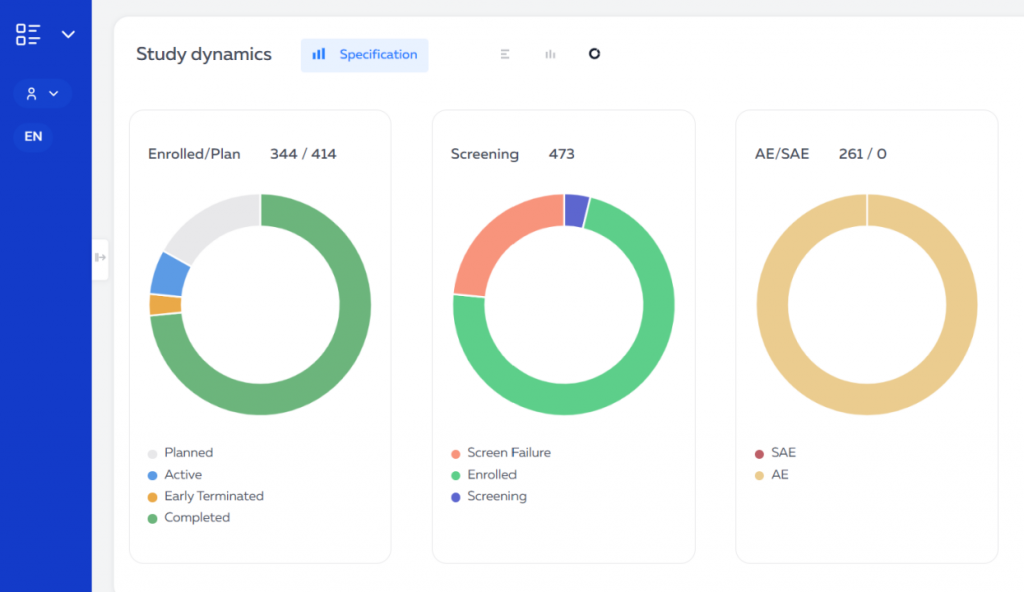
Aggregated data are summarized information that can be useful for quickly reviewing the progress of a study and identifying general trends.
Thanks to the new service, employees of sponsoring companies are able to focus on discussing specific issues with the project manager, rather than spending time searching for and analyzing information about the progress of the study.
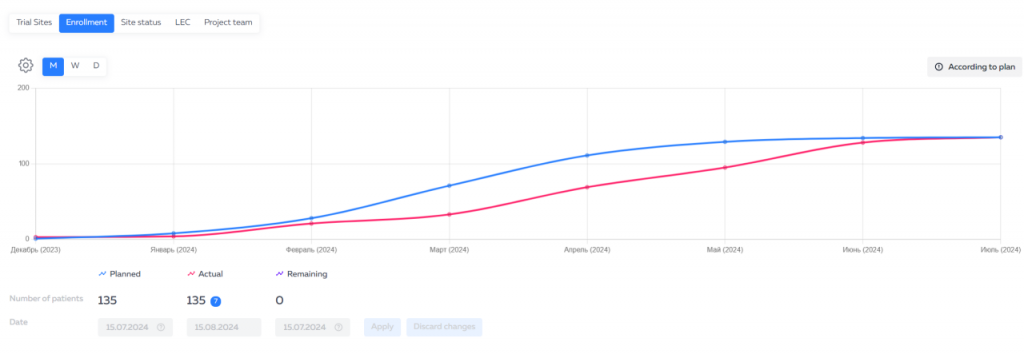
This innovative solution from IPHARMA significantly simplifies the process of controlling clinical trials and increases the efficiency of the sponsoring companies’ employees. The service has already received positive feedback from the first users and continues to evolve to provide even more convenient tools for trial management.
How does the service work?
Employees of the sponsoring companies have access to the platform through a web interface. They can view various indicators of study progress, such as enrollment, adverse events, responses to inquiries, and completion of individual enrollment cards.
For ease of use, data is presented in dashboards – dashboards that provide a quick overview of study progress on key indicators. Dashboards can be customized to your needs by selecting the necessary indicators and filters.
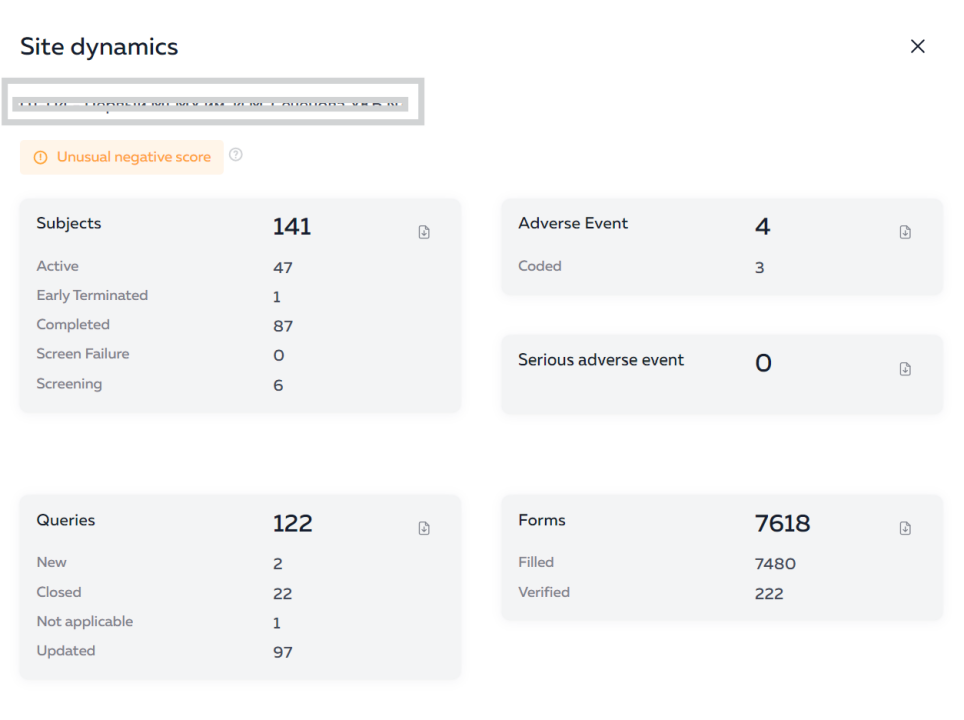
Thus, the new service from IPHARMA is a convenient tool for monitoring the progress of clinical trials and increasing the efficiency of the employees of sponsor companies.