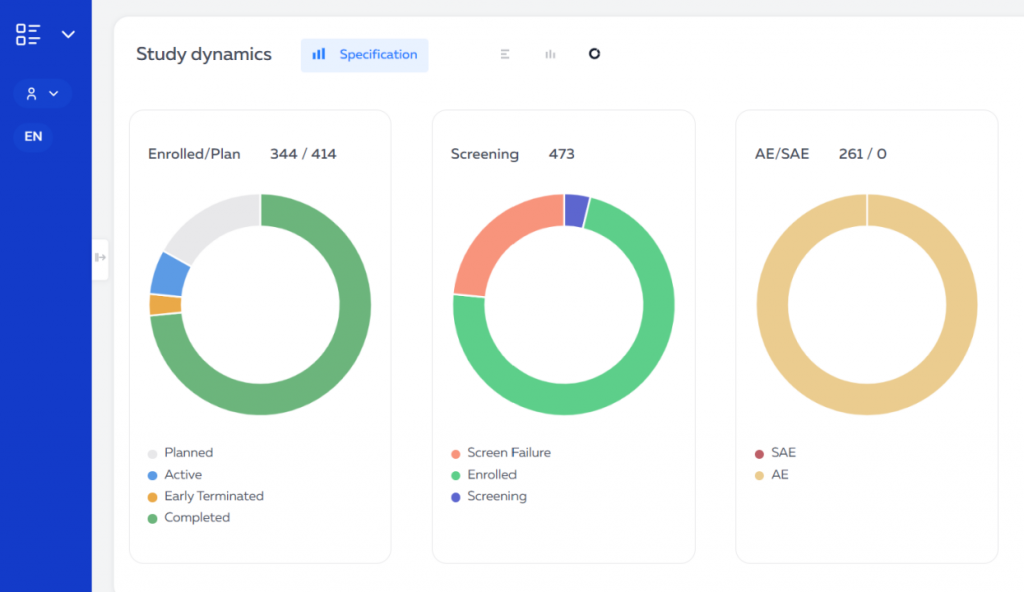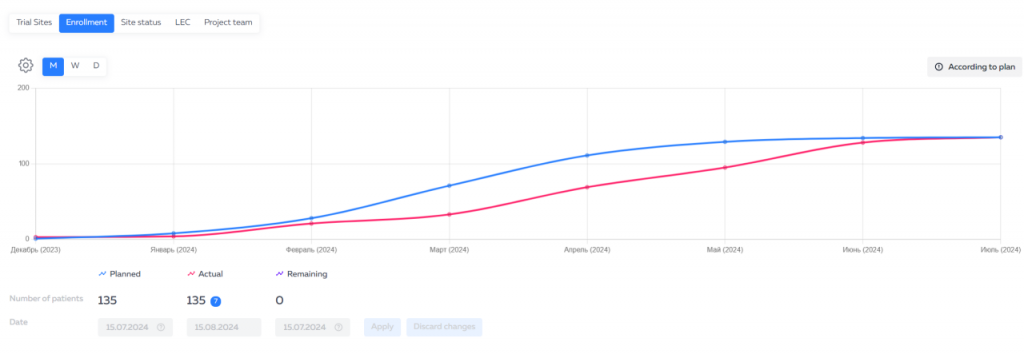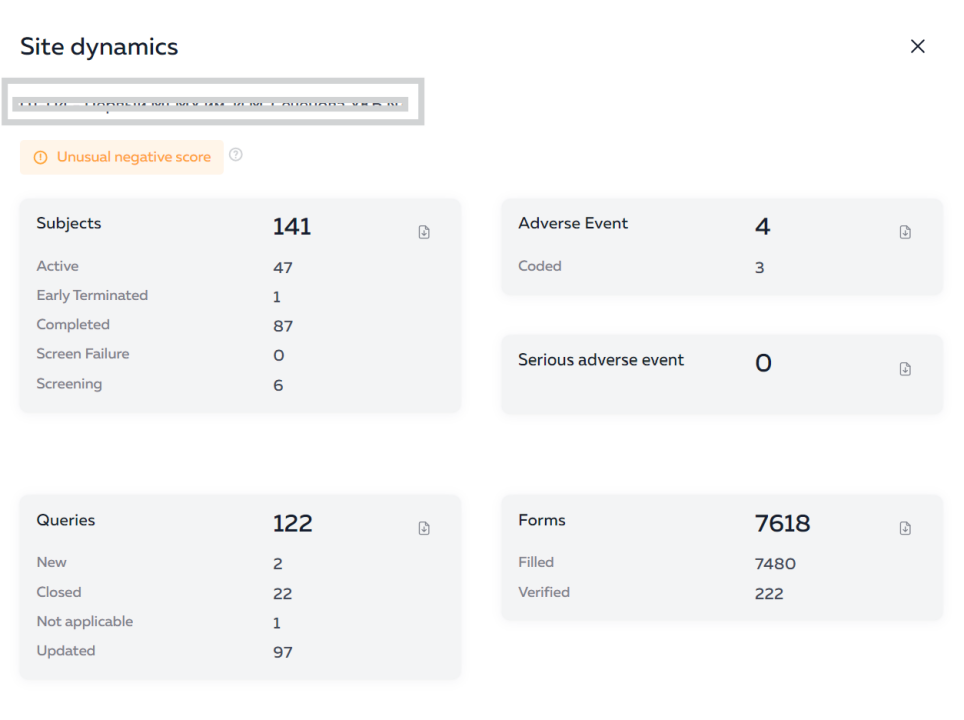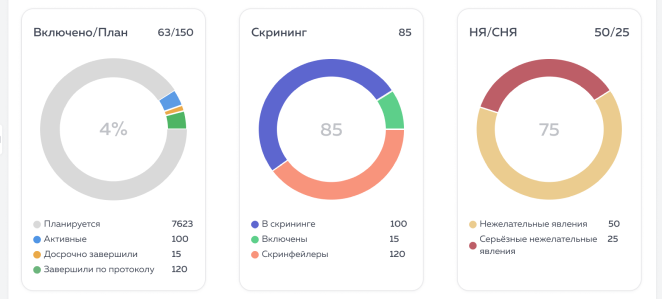
The Eurasian Economic Union (EEU or EAEU) continues to improve legislation for conducting clinical trials and registering medicines on its territory.
There are leaders in the region in terms of the number of clinical trials conducted, such as Russia and Belarus. These countries have a large number of experienced research sites, convenient regulatory environment and developed infrastructure for conducting clinical trials.
In addition, there are a number of countries where the clinical trials market is in the initial growth stage. Due to harmonization of processes between countries, this market is expected to grow strongly.
The region has a distinct advantage over other countries in conducting clinical trials. Russia and Belarus remain leaders in the EAEU in terms of the number of trials conducted due to the following factors: large patient pool, low costs for services and remuneration of the investigators, favorable regulatory environment, high qualification of investigative teams and clinical trial specialists working in CROs and sponsor companies.
Let’s take a closer look at these advantages:
- Availability of a patient pool. EEU countries have a centralized healthcare system, so patients with the same diseases are seen in specialized hospitals, which makes it easier to find them. At the same time, patients have high adherence to follow the procedures of the clinical trial protocol because of regular medical check-ups. The limited capacity of the healthcare system for certain diseases, such as oncology and orphan diseases, also plays a role.
- A large number of experienced investigative teams. EEU countries have teams with experience in conducting international clinical trials and passing FDA and EMA inspections. The cost of services and remuneration of medical professionals and investigators in these countries is significantly lower than in Western Europe and the USA.
- Favorable regulatory environment. For example, in Russia, taking into account the planned changes in legislation, it takes 31 working days to obtain approval to conduct a clinical trial, and up to 5 working days to obtain permission to import an investigational drug and export biosamples. From the moment documents are submitted to the Ministry of Health until the first patient is included in the study, it can take only 3 months, which is quite common.
- Developed medical infrastructure. The region has research sites capable of conducting complex clinical trials that include a large number of diagnostic and invasive procedures. This is especially true for such complex studies as oncology, oncohematology and autoimmune diseases.
Statistics from international clinical trials in Russian (before 2022) confirm these advantages:
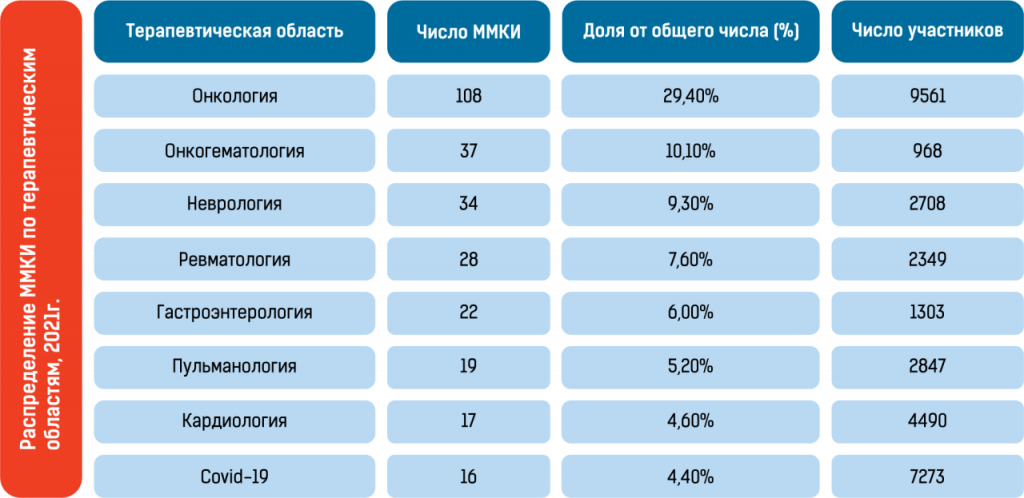
In summary, the availability of a large patient pool, a centralized healthcare system, high patient adherence to the protocol, large research sites with advanced infrastructure, and short approval periods for clinical trials allow for faster patient recruitment and shorter overall clinical development timelines.